Dr. Hannah Pizzato, a scientist at the U of A Health Sciences Center for Advanced Molecular and Immunological Therapies, is working on a stem cell-based therapy for Type 1 diabetes.
People have been waiting a long time for a cure for Type 1 diabetes. Hannah Pizzato, PhD, is one of them.

“My whole life I was told a cure is coming and that I won’t have to deal with this very long, but then a cure never came,” said Pizzato, a principal scientist at the University of Arizona Health Sciences Center for Advanced Molecular and Immunological Therapies.
Pizzato was diagnosed with Type 1 diabetes when she was 4 years old. She was too young to understand it is an autoimmune disease. She didn’t know her immune system was attacking cells in her pancreas that are responsible for producing insulin, a hormone that regulates blood sugar. But from an early age, she began to question why people become sick.
“I was always interested in the fundamentals of how the body works and adapts over time to keep us alive,” she said. “I was intrigued by how biology influences human development.”
It isn’t a surprise her educational and research trajectory focused on the immune system and Type 1 diabetes. She received her doctorate from Washington University in St. Louis, where she worked under the mentorship of Deepta Bhattacharya, PhD, who recently was named the inaugural executive director of CAMI.
In 2017, Pizzato followed Bhattacharya to Tucson, where she finished her thesis and became a postdoctoral fellow in his lab at the U of A College of Medicine—Tucson Department of Immunobiology.
In Bhattacharya’s lab, Pizzato worked to overcome immune barriers to stem cell transplantation. Today, as one of the first scientists working at CAMI, Pizzato is building upon that research with the goal of one day using a stem cell therapy to finally cure Type 1 diabetes.
Building toward a cure
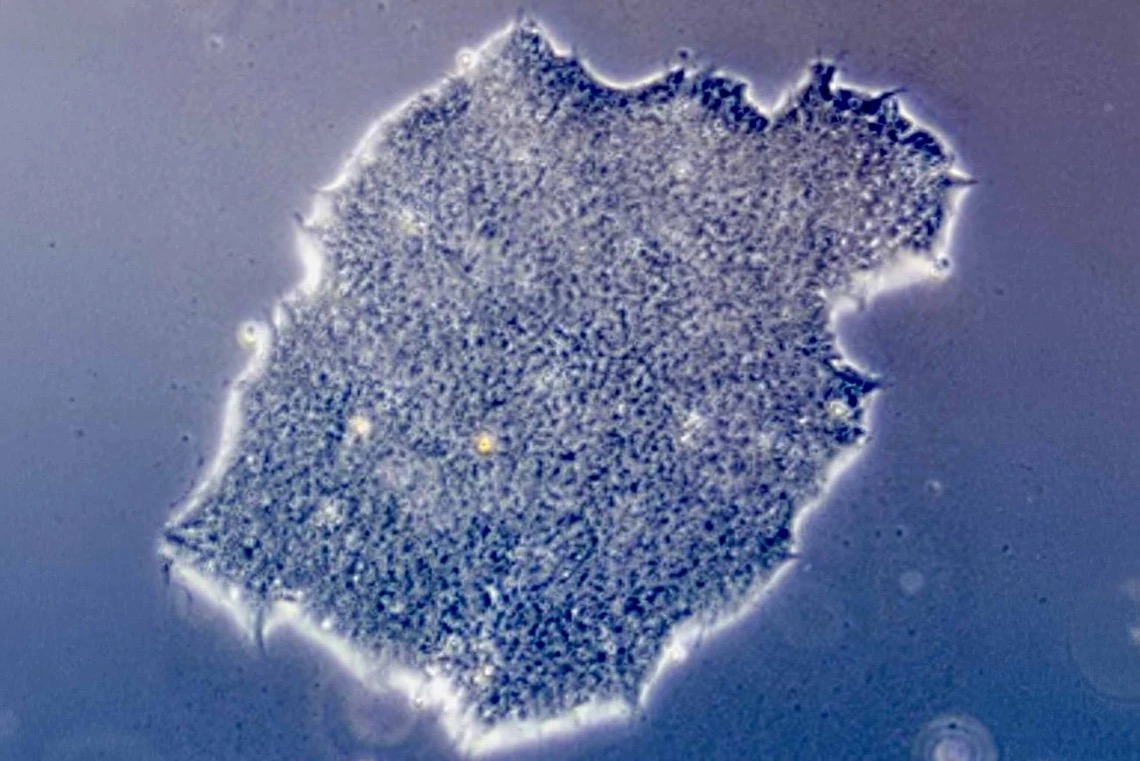
Pizzato’s interest in the immune system brought her to the forefront of a field that is pushing the boundaries of modern medicine: stem cell therapy. Stem cells are unique in that they are self-replicating and unspecialized, meaning they can turn into many different types of cells in the body, including blood, muscle and brain cells.

Since the first successful bone marrow transplant in 1956, scientists all over the world have been examining stem cells and their potential to fight diseases, regenerate damaged tissues and develop personalized treatments for patients. Today, hematopoietic stem cells found in bone marrow are used to treat blood cancers such as leukemia, lymphoma, multiple myeloma and more.
One of the fastest-growing areas of stem cell research utilizes pluripotent stem cells, which are cells that can turn into any cell type in the body.
“We are researching pluripotent stem cells because they can be differentiated into other kinds of cells that perform specialized functions,” Pizzato said. “In theory, these stem cells could be transformed into anything a patient might need. For example, our goal is to mature stem cells into insulin-producing beta cells and transplant them into someone with Type 1 diabetes. We could replace the beta cells they lost and reverse the disease.”
The biggest challenge to stem cell therapies comes from the immune system, the body’s natural protector against invaders. This complex network of cells, tissues and organs fights off infections, diseases and, unfortunately, regenerative treatments including stem cells.
“In the same way that the body’s immune system will reject a kidney transplant, the body will reject stem cells without the assistance of immunosuppressive drugs,” Pizzato explained. “But lifelong immunosuppression increases your risk of infections and can even allow cancer cells to form and proliferate unchecked.”
Bhattacharya and Pizzato have spent years trying to find a solution to this problem. Now, they may be on the verge of a breakthrough.
“We think modifying stem cells in a way that hides them from the immune system can avoid the need for immunosuppression and potentially make for a really effective therapy,” Pizzato said.
Engineering a disguise for stem cells
Pizzato was the first author on a paper published in Stem Cell Reports earlier this year. In the study, the research team genetically engineered stem cells to evade detection by various parts of the immune system, including T cells.
T cells, a type of white blood cell, are key drivers of immune rejection. They are constantly scanning for anything that doesn’t belong in the body, such as viruses and bacteria. T cells even perform security checks on other cells by checking for human leukocyte antigen, a protein found on the surface of most cells. If that protein isn’t a match to the body’s specific human leukocyte antigen, the T cell is triggered to kill that cell.

“The human leukocyte antigen on transplanted cells is a dead giveaway to T cells that they are not part of the body,” she said. “To get around this problem, we used a genetic engineering tool to cut out the gene that encodes for that protein. This acts as a camouflage to get our modified stem cells around any investigating T cells.”
The lack of human leukocyte antigen solved the T cell problem, but it didn’t address two other parts of the immune system: natural killer cells and complement deposition. Natural killer cells, like T cells, are always on the lookout to destroy damaged or diseased cells. The immune system also relies on a process called complement deposition, where certain proteins bind to the surface of invaders to help the immune system recognize threats.
Pizzato said that after removing human leukocyte antigen, the team added several other proteins, some of which sent inhibitory signals to hinder the natural killer cells and others preventing complement deposition.
“It’s like we took the stem cell’s glasses off, and then added a hat, fake moustache and coat to prevent recognition,” Pizzato said.
The modified stem cells were tested in mice with fully functioning immune systems, where they successfully dodged rejection by the immune system and persisted.
“These findings are very encouraging and give us confidence we are ready to move forward,” Pizzato said.
Moving forward at CAMI
In July, Pizzato moved to Phoenix to set up the first CAMI lab on the second floor of the Biomedical Sciences Partnership Building.
Some of the first experiments Pizzato has planned include narrowing down the number of modifications needed to successfully disguise stem cells from healthy immune systems. Eventually, she and Bhattacharya hope to test the engineered stem cells in nonobese diabetic mouse models of Type 1 diabetes and translate their mouse work into human findings.
“This will be an especially important step and a big test for our modified stem cells,” Pizzato said. “Type 1 diabetes is an autoimmune disease, so the immune system is already primed to be a hostile environment. The first big step was making stem cells that are invisible to the immune system, but invisible, unspecialized stem cells floating around the body don’t really help anyone. We want to turn those invisible stem cells into beta cells and make sure they can help regulate blood sugar.”
For Pizzato, CAMI is helping bridge the gap between her research and a potential product. It might also help a lifelong dream come true.
By Brian Brennan, U of A Health Sciences Office of Communications